Case Quiz (January 2017)
A girl aged 12 years with no previous morbidity was referred with a 2-week history of evident periorbital and pretibial edema, myalgia, and fatigue. Her blood pressure was 130/80 mm Hg, and urine dipstick testing showed proteinuria +++ and hematuria ++.
She had no recent history of infections and she was not taking any medication. She had a β-thalassemic trait. Her family history was unremarkable for renal diseases but positive for thrombosis.
Laboratory investigations revealed mild renal failure with serum creatinine at 1.24 mg/dl, hemoglobin at 10.1 g/dl, and a normal platelet count. Serum electrolytes, albumin, and the lipid panel were within the normal range. Urine analysis showed proteinuria up to 2 g/24 h and presence of 107 erythrocytes/μl. Renal ultrasound was unremarkable. The complement system components C3 and C4, hemolysis indices, antinuclear and antineutrophil cytoplasmic antibodies, prothrombin time, and activated partial thromboplastin time were normal. Viral serologic markers for hepatitis B, hep- atitis C, varicella, cytomegalovirus, Epstein-Barr virus, rubella, measles, and mumps excluded any infection. Thrombophilia screening tests showed mild hyperhomocysteinemia up to 15.5 μmol/l (normal value 4–13) and a methylenetetrahydrofolate reductase (MTHFR) gene homozygous mutation (C677T variant).
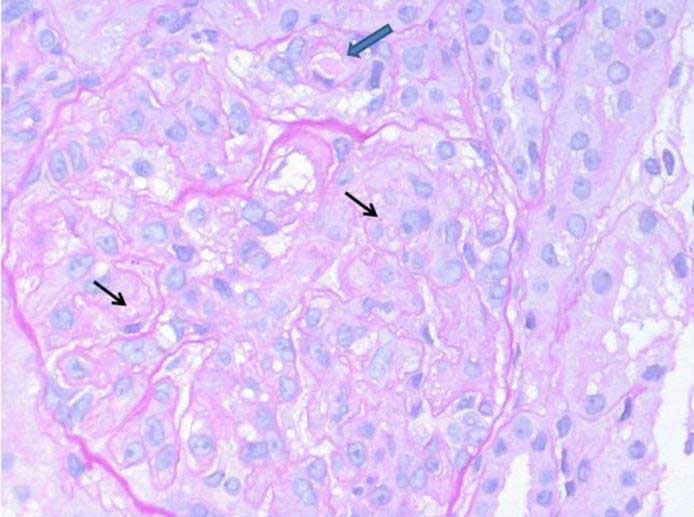
Renal biopsy was done and abnormal findings were seen (see figure). A routine immunofluorescence panel (IgA, IgG, IgM, C1q, C3, and fibrinogen) was substantially negative in glomeruli; C3 complement fraction stained arteriolar walls
Case Answer (January 2017)
aHUS is a genetic, chronic, and progressive inflammatory disease that represents 5–10% of pediatric cases of HUS.Extensive research has established an association between aHUS and dysregulation of the alternative complement pathway. Although 50% of patients have a heterozygous loss of function mutation in a regulatory gene such as CFH, CFI, or MCP, a gain of function mutation in an effector gene such as CFB or C3 and acquired CFH antibodies have been identified in patients with both familial and sporadic aHUS. More recently, mutations in the Thrombomodulin (THBD) gene have been associated with aHUS in about 5% of patients.
Thrombomodulin is a ubiquitous transmembrane endothelial cell glycoprotein with anticoagulant, anti-inflammatory, and cytoprotective properties. Thrombomodulin, in the presence of cofactors such as CFH or C4b-binding protein, negatively regulates complement by accelerating factor I-mediated inactivation of C3b. By binding to thrombin – thereby preventing it from activating C5 – and by promoting activation of the plasma procarboxypeptidase B, thrombomodulin also accelerates the inactivation of anaphylatoxins C3a and C5a, providing additional protection of the membrane surface.
In our patient, a carrier of the THBD P501 variant, disease onset was around the age of 14 years with specific clinical manifestations. The thermolabile variant (C677T) of MTHFR does not seem to be a significant risk factor for venous thromboembolic events, the coexistence in our patient of THBD abnormalities and moderate hyperhomocysteinemia may potentiate a systemic risk of thrombotic and ischemic events, worsening disease prognosis.