Case Quiz (March 2016)
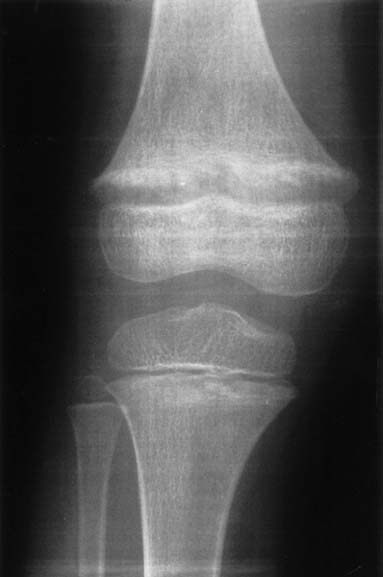
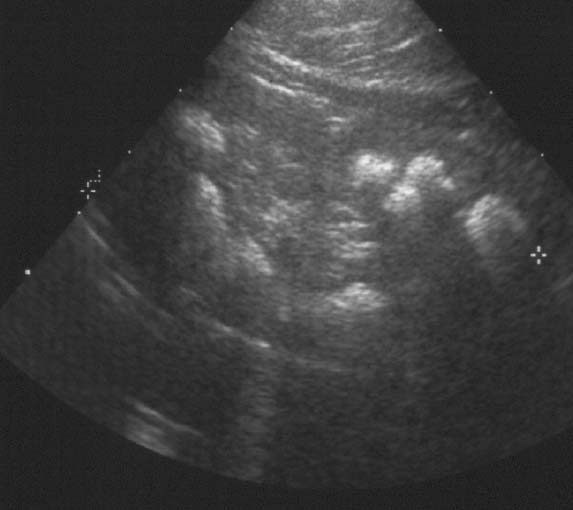
A 5-year-old girl was referred for further diagnostic work-up of rickets and bilateral nephrocalcinosis. She is the first child of healthy unrelated parents. She was born after an uneventful pregnancyat 40 weeks gestation, with a body weight of 2,680 g. Body height and weight remained below the 3rd percentile,she was reported to drink a lot, and psychomotor development was normal. When investigations were carried out for short stature,rickets and bilateral medullary nephrocalcinosis were observed by both U/S and X-ray. The girl has two sisters, aged3 years 6 months and 1 year 6 months, respectively, who are in good health to date. At presentation, body height was 96 cm and weight 13 kg. Blood pressure was 106/50 mmHg. Physical examinationwas normal except for large wrists, moderate genu valgum,and mild femoral bowing.Blood chemistry yielded the following information: urea (52 mg/dl) and creatinine (0.8 mg/dl), total calcium (10.7 mg/dl), magnesium(1.8 mEq/l), phosphate (1.6 mg/dl), and alkaline phosphatase (909 IU/l), parathyroid hormone (PTH) (<0.2 pg/ml) (normal 10–60) and 1,25-(OH)2 vitamin D (79 pg/ml) (normal 18–45). Serum electrolytes,albumin, transaminases, and uric acid were within normal ranges.Urine microscopy showed white cells but no erythrocytes. A24-h urine collection yielded 430 mg of protein per 1.73 m2 and 6.2 mg/kg calcium; excretion of uric acid, amino acids, and oxalate were normal and there was no glucosuria. Urinary β2 microglobulin/creatinine ratio was 1,312 μg/g. The maximal urinary osmolality was 304 mosmol/kg water under DDAVP stimulation. Bone X-rays showed metaphyseal lesionstypical for rickets (Fig. 1); bone age was 5 years. Renal U/S confirmed pronounced bilateral medullary nephrocalcinosis. The patient had been investigated at the age of 1 year because of poor weight gain. At that time, serum calcium was 12.1 mg/dl,phosphate was 3.9 mg/dl, and serum creatinine 0.6 mg/dl. She hadtaken vitamin D supplements (300 IU daily) up to the day of diagnosis.
Case Answer (March 2016)
Our patient is likely to have (Hereditary Hypophosphatemic rickets with Hypercalciuria) HHRH, since she has allthe hallmarks of the disorder: renal phosphate leak, depressedPTH, high serum calcitriol, and hypercalciuria.
However, there are special features that make the diagnosissomewhat equivocal: proteinuria, pronounced nephrocalcinosis, which has never been reported in associationwith HHRH, and a reduced GFR.
The fact that she has tubular proteinuria suggests Dent disease.
Dent disease is another genetic disordercharacterized mainly by hypercalciuria and tubular protinuria. The disease is also known as “familial idiopathiclow molecular weight proteinuria” in Japan andas “X-linked nephrolithiasis” in the United States. Dent disease is caused by inactivating mutations of the chloridechannel ClC-5 expressed in the endosomes of theproximal tubular epithelium. The knock-out mouse lacking the ClC-5 gene has low molecular weight proteinuria,generalized aminoaciduria, glucosuria, and hypercalciuria with calcium deposits in the kidneys.The clinical manifestations in humans differ from one individual to another and from one family to another and can include renal phosphate wasting, resulting in clinical and radiological signs of rickets. The mode of inheritance—X-linked recessive—indicates that boys are more severely affected than girls. Our patientis a girl and if she were affected by this condition,the severity in terms of nephrocalcinosis and reduced GFR would be quite surprising. Moreover, her proteinuriais only partially tubular in origin and could be the consequence of nephrocalcinosis. Therefore, Dent diseasein our patient is unlikely.