Case Quiz (March 2020)
A 7-year-old girl with no significant past medical or family history presented with decreased urine output and dyspnea for 3 days and with history of fever, rash, myalgia, vomiting and diarrhoea for 3 weeks. She was tachycardic, hypertensive, with lower limb edema and had decreased breath sounds at right lung base with evidence of pleural and pericardial effusions.
Investigations revealed serum creatinine 3 mg/dL, haemoglobin 5 g/dL, platelets 14.x109 /l, leucocytosis and eosinophilia , with presence of schistocytes (10%). She had elevated LDH and CRP, low haptoglobin and serum ferritin 5269 ng/ mL. Her serum albumin was low with elevated transaminases and triglycerides. Her rheumatological serum markers were negative with normal complements C3/C4 and fibrinogen. Urinalysis showed microscopic hematuria and mild proteinuria and stool studies were negative including that of Shiga toxin. The ADAMTS13 activity was 56%.
Her genetic testing was abnormal for: (1) large CRHR1–CFHR3 homozygous deletion (factor H autoantibody test was negative); (2) Silent variant (c.315A>T, p.Thr105Thr) of unknown significance in exon 2 of Complement Factor I; (3) heterozygous silent variant (c.3753C>A, p.Pro1251Pro) of unknown significance in exon 29 of C3; (4) heterozygous missense variant (c.59G>A, p.Gly20Glu) in exon 2 of DGKE; (5) heterozygous polymorphism (IVS978G>A) within an intron in Membrane Cofactor Protein/CD46 .
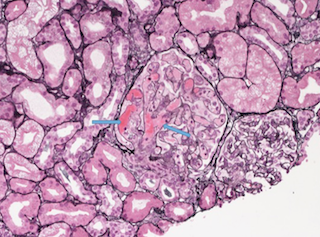
Renal biopsy was done
Case Answer (March 2020)
Diacylglycerol kinase-E (DGKE) gene mutation may result in aHUS and membranoproliferative-like glomerular thrombotic microangiopathy. The onset of aHUS is typically in the first years of life, however, there are reports of intronic DGKE mutation (c.888+40A>G) that segregated with disease. Our patient’s heterozygous missense variant in exon 2 of DGKE, found more commonly in Africans
HLH was considered in our patient because of anaemia, thrombocytopenia, high acute-phase reactants including very high ferritin and elevated transaminases but was unsupported by absence of splenomegaly, rash and lack of systemic infection, immunodeficiency or underlying malignancy.
CFHR1-CFHR3 homozygous deletion may be present in 5%–15% of the general population, and even as high as 33% in some populations of African descent.