Case Quiz (November 2022)
girl aged 11 years with no previous morbidity was referred with a 3-week history of evident periorbital and lower limb edema, myalgia, and fatigue. Her blood pressure was 130/80 mm Hg, and urine dipstick testing showed heavy range proteinuria (+++) and microscopic hematuria (60-70/HPF). She had no recent history of infections and she was not taking any medication. Her family history was unremarkable for renal diseases but positive for thrombosis.
Laboratory investigations revealed mild renal impairment with serum creatinine at 1.12 mg/dl, hemoglobin at 9.8 g/dl, and a normal platelet count. Serum electrolytes, albumin, and the lipid panel were within the normal range. Urine analysis showed proteinuria up to 2.5 g/24 h and presence of 209 erythrocytes/μl. Renal U/S was unremarkable. The complement system components C3 and C4, hemolysis indices, antinuclear and antineutrophil cytoplasmic antibodies, prothrombin time, and activated partial thromboplastin time were normal. Viral serologic markers for hepatitis B, hepatitis C, varicella, cytomegalovirus, Epstein-Barr virus, rubella, measles, and mumps excluded any infection. Thrombophilia screening tests showed mild hyperhomocysteinemia up to 15.5 μmol/l (normal value 4–13) and a methylenetetrahydrofolate reductase (MTHFR) gene homozygous mutation (C677T variant).
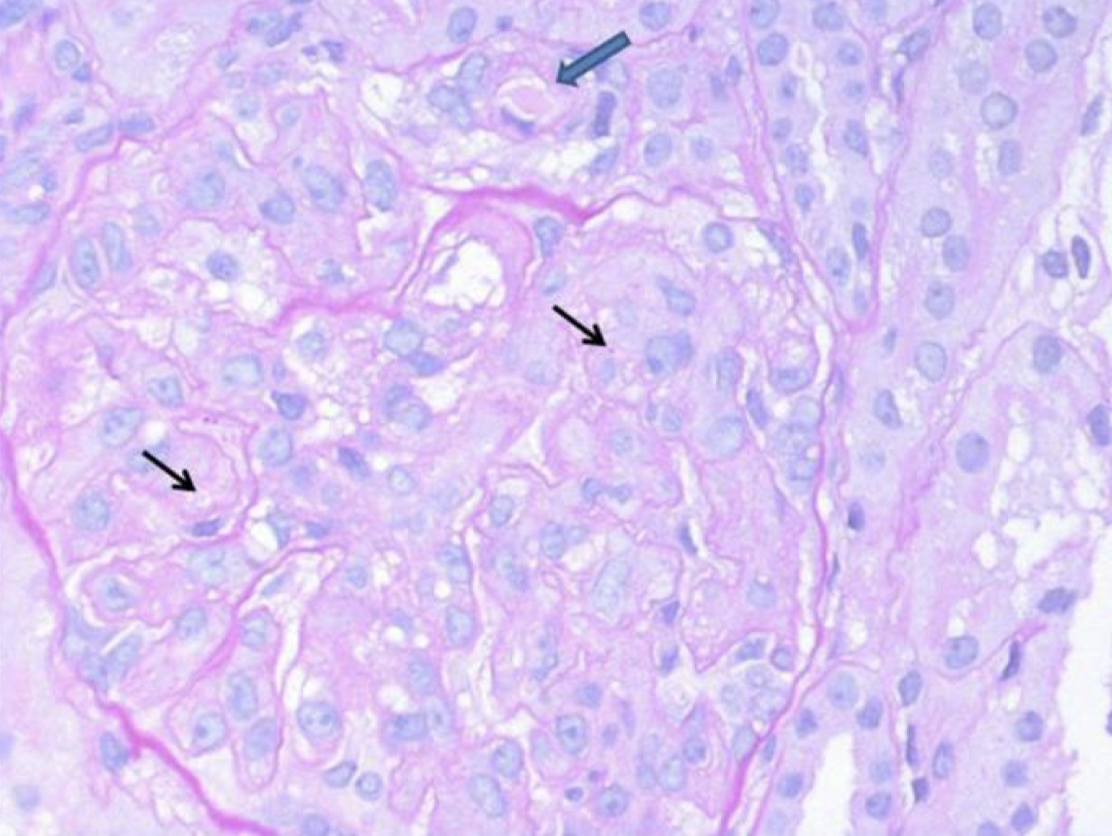
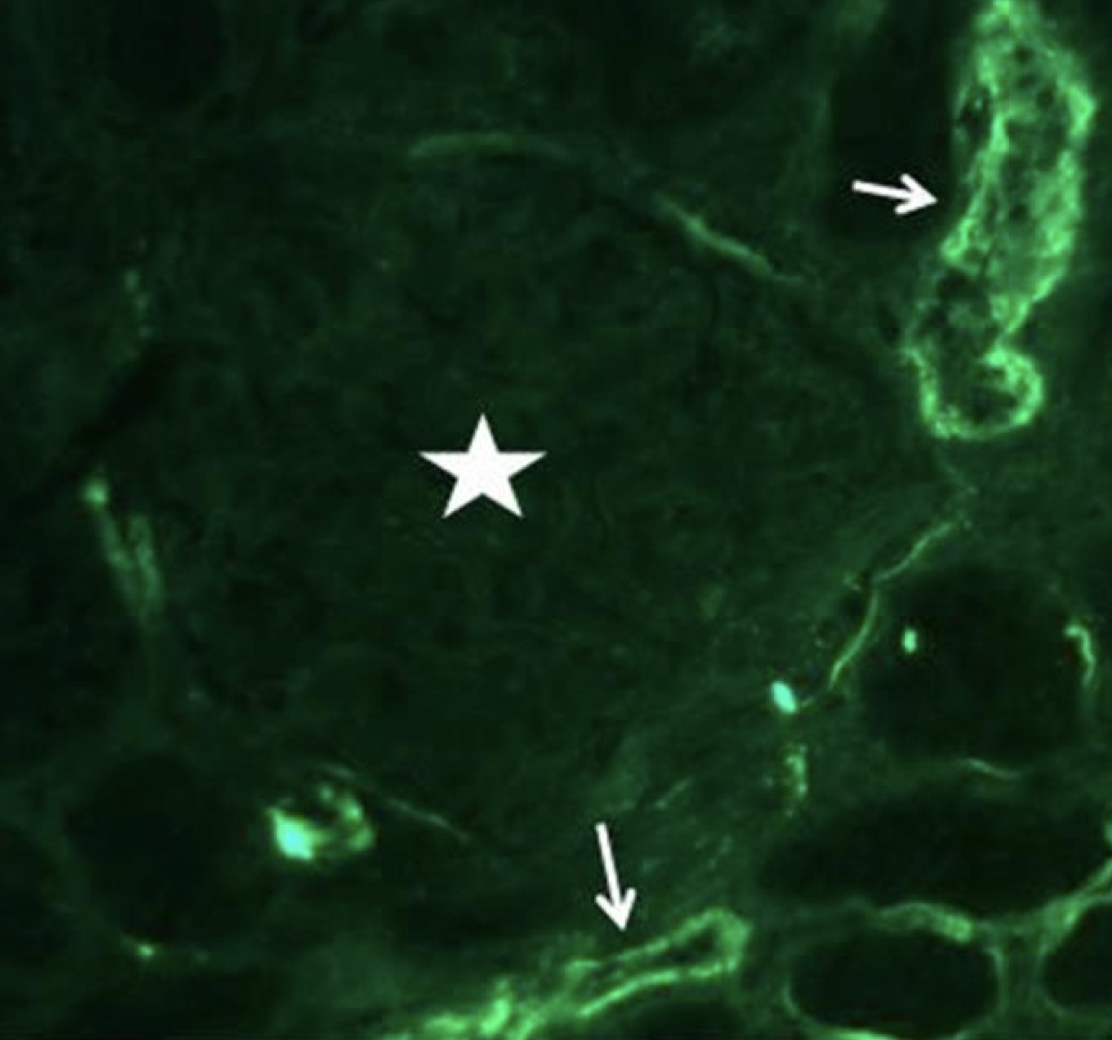
Renal biopsy demonstrated diffuse thickening and multilayering of glomerular capillaries with entrapment of red blood cell fragments. Hilar arterioles showed intimal fibromucoid hyperplasia; occasional thrombi occluded vascular lumina (figure). I/F panel for IgA, IgG, IgM, C1q, C3, and fibrinogen was substantially negative in glomeruli (figure). These findings were consistent with thrombotic microangiopathy (TMA).
Thrombotic thrombocytopenic purpura was excluded by normal ADAMT13 and antibodies against ADAMTS13.
A low-sodium diet, and antihypertensive therapy with carvedilol and amlodipine were started with normalization of blood pressure. When the nephrosis had resolved, captopril was introduced to control proteinuria. A thrombophilic status was treated by cobalamin and acetylsalicylic acid administration. Within 2 months, the proteinuria became negative. The patient discharged with creatinine 1.04 mg/dl.
Nine months after disease onset, the patient presented severe microangiopathic hemolytic anemia [hemoglobin 7.4 g/dl, lactate dehydrogenase (LDH) increased to 1350 U/l, haptoglobin <1 mg/dl, and reticulocytes 8%] with a normal platelet count (176,000/mm3 ). Serum creatinine was 1.24. Circulating C3, C4, and C5b-9 levels were normal.
A diagnostic test was made
Case Answer (November 2022)
Thrombomodulin gene mutation causing aHUS
Thrombomodulin is a ubiquitous transmembrane endothelial cell glycoprotein with anticoagulant, anti-inflammatory, and cytoprotective properties.Thrombomodulin, in the presence of cofactors such as CFH or C4b-binding protein, negatively regulates complement by accelerating factor I-mediated inactivation of C3b. By binding to thrombin – thereby preventing it from activating C5
Rare mutations of thrombomodulin expression predisposes to the endothelial injury and microvascular thrombosis typical of aHUS.